Publications
27.
26.
25.
24.
23.
22.
21.
20.
19.
18.
17.
16.
15.
14.
13.
12.
11.
10.
9.
8.
7.
6.
5.
4.
3.
2.
1.
Development of MDM2-Targeting PROTAC for Advancing Bone Regeneration.
Jeong, S.†; Cha, J.-K.†; Ahmed, W.†; Kim, J.; Kim, M.; Hong, K. T.; Choi, W.; Choi, S.; Yoo, T. H.; An, H.-J.; An, C. S.; Lee, J.; Choi, J.; Kim, S.-Y.; Lee, J.-S.; Lee, S.*; Choi, J.*, Kim, J. M.* († contributed equally)
Adv. Sci. ASAP. DOI: 10.1002/advs.202415626
Therapeutic Potential of Polydopamine-Coated Selenium Nanoparticles in Osteoarthritis Treatment.
Park, K.-C.†; Choi, J.†; Choi, S.; Lee, G.; An, H.-J.; Yun, H.; Lee, S.* Int. J. Pharm. 2025, 675, 125568 († contributed equally).
DOI: 10.1016/j.ijpharm.2025.125568
Discovery of Orally Bioavailable Phthalazinone Analogues as an ENPP1 Inhibitor for STING-Mediated Cancer Immunotherapy
Cho, Y.; Kang, M.; Ji, S. H.; Jeong, H. J.; Jung, J. E.; Oh, D. H.; Park, S.; Park, Y.-Y.; Choi, J.; Kim, S.; Kim, N.-J.; Lee, D.-H; Park, C. S.*; Han, S.-J.*; Lee, S.*; Choi, J.*
J. Med. Chem. 2023, 66, 15141. DOI:10.1021/acs.jmedchem.3c01061
N4-phenylquinazoline-4,6-diamine as a tunable fluorescent scaffold for the development of fluorescent probes.
Lee, J.; Choi, S.-K.; Aslama, Lim, W. Lee, J.; Ko, J.; Ryu, C. H.; Lee, K. M.; Jung, Y. M.; Yoo, H. S.; Park, J. H.; Lee, S.; Choi, J.; Kim, E.*; Park, J.*
Dyes Pigm. 2023, 210, 110987. DOI: 10.1016/j.dyepig.2022.110987
Small molecule ectonucleotide pyrophosphatase/phosphodiesterase 1 inhibitors in cancer immunotherapy for harnessing innate immunity.
Choi, J.*
Bull. Korean Chem. Soc. 2023, 44, 88–99. DOI: 10.1002/bkcs.1264612
Effect of the Duration of NSAID Use on COVID-19.
Kim, K.; Yoon, S.; Choi, J.; Lee, S.*
Medicina 2022, 58, 1713. DOI: 10.3390/medicina58121713
Finite element analyses of lateral condyle fracture fixation in paediatrics regarding configuration of Kirschner-wire.
Jeon, S.; Ahn, W.; Oh, J.; Chung, J.; Choi, J.; Lee, S.*
BMC Musculoskeletal Disord. 2022, 23, 940. DOI: 10.1186/s12891-022-05897-3
The Association of Low Skeletal Muscle Mass with Complex Distal Radius Fracture.
Oh, C.-H.; Kim, J.; Kim, J.; Yoon, S.; Jung, Y.; Lee, H. I.; Choi, J.; Lee, S.*; Han, S.-H.*
J. Clin. Med. 2022, 11, 5581. DOI: doi.org/10.3390/jcm11195581
miR-31-3p functions as a tumor suppressor by directly targeting GABBR2 in prostate cancer.
Choi, S.†; Lee, S.†; Han, Y.-H.; Choi, J.; Kim, I.; Lee, J.; An, H.-J.* († contributed equally)
Front. Oncol. 2022, 12, 945057. DOI: doi.org/10.3389/fonc.2022.945057
Colchicine as a novel drug for the treatment of osteosarcoma through drug repositioning based on an FDA drug library.
Park, K.-C.; Kim, K. S.; Jung, B. S.; Yoon, S.; Ahn, W.; Jeong, S.; Choi, J.; Lee, S.*
Front. Oncol. 2022, 12, 893951. DOI: 10.3389/fonc.2022.893951
Hair methylmercury levels are inversely correlated with arterial stiffness.
Park, K.-C.; Kim, K. S.; Jung, B. S.; Yoon, S.; Ahn, W.; Jeong, S.; Choi, J.; Lee, S.*
Atherosclerosis 2022, 357, 14–19. DOI: 10.1016/j.atherosclerosis.2022.08.003
Synthesis of Benzoxaphosphole 1-Oxide Heterocycles via a Three-Component Coupling Reaction Involving Arynes, Phosphites, and Ketones
Jeong, H. J.†; Kim, J. H.†; Lee, J. K.; Yoon, H. J.; Choi, J.*; Han, S.-J.* († contributed equally)
Org. Lett. 2022, 24, 2192−2196. DOI: 10.1021/acs.orglett.2c00515
Harnessing aggregation-induced emission property of indolizine derivative as a fluorogenic bioprobe for endoplasmic reticulum.
Arun, V.; Choi, S.-K.; Han, J. H.; Choi, H.; Kim, H.-M.; Kim, W.; Choi, J.*; Kim, J.*; Kim, E.*
Dyes Pigm. 2022, 200, 110118. DOI: 10.1016/j.dyepig.2022.110118
Fluorescent sensor array for high-precision pH classification with machine learning-supported mobile devices.
Kim, H.; Lee, S.; Min, J. S.; Kim, E.; Choi, J.; Ko, J. G.*; Kim, E.*
Dyes Pigm. 2021, 193, 109492. DOI: 10.1016/j.dyepig.2021.109492
Benefits of Chemical Sugar Modifications Introduced by Click Chemistry for Glycoproteomic Analyses.
Calle, B.; Bineva-Todd, G.; Marchesi, A.; Flynn, H.; Ghirardello, M; Tastan, O. Y.; Roustan, C.; Choi, J.; Galan, M. C.; Schumann, B.*; Malaker, S. A.*
J. Am. Soc. Mass Spectrom. 2021, 32, 2366−2375. DOI:10.1021/jasms.1c00084
Optimization of Metabolic Oligosaccharide Engineering with Ac4GalNAlk and Ac4GlcNAlk by an Engineered Pyrophosphorylase.
Cioce, A; Bineva-Todd, G.; Agbay, A. J.; Choi, J.; Wood, T. M.; Debets, M. F.; Browne, W. M.; Douglas, H. L.; Roustan, C.; Tastan, O. Y.; Kjaer, S.; Bush, J. T.; Bertozzi, C. R.; Schumann, B.*
ACS Chem. Biol. 2021, 16, 10, 1961–1967. DOI:10.1021/acschembio.1c00034
Metabolic precision labeling enables selective probing of O-linked N-acetylgalactosamine glycosylation.
Debets, M. F.; Tastan, O. Y.; Wisnovsky, S. P.; Malaker, S. A.; Angelis, N.; Moeckl, L. K. R.; Choi, J.; Flynn, H.; Wagner, L. J. S.; Bineva-Todd, G.; Antonopoulos, A.; Cioce, A.; Browne, W. M.; Li, Z.; Briggs, D. C.; Douglas, H. L.; Hess, G. T.; Agbay, A. J.; Roustan, C.; Kjaer, S.; Haslam, S. M.; Snijders, A. P.; Bassik, M. C.; Moerner, W. E.; Li, V. S. W.; Bertozzi, C. R.; Schumann, B.*
Proc. Natl. Acad. Sci. U. S. A. 2020, 117, 25293−25301. DOI: 10.1073/pnas.2007297117
Bump-and-Hole Engineering Identifies Specific Substrates of Glycosyltransferases in Living Cells.
Schumann, B.*; Malaker, S. A.; Wisnovsky, S. P.; Debets, M. F.; Agbay, A. J.; Fernandez, D.; Wagner, L. J. S.; Lin, L.; Li, Z.; Choi, J.; Fox, D. M.; Peh, J.; Gray, M. A.; Pedram, K.; Kohler, J. J.; Mrksich, M.; Bertozzi C. R.*
Mol. Cell 2020, 78, 824−883. DOI: 10.1016/j.molcel.2020.03.030
Phase Behavior of Disk Coil Block Copolymers under Cylindrical Confinement: Curvature Induced Structural Frustrations.
Ha, M. Y.†; Ryu, J. H.†; Cho, E. N.; Choi, J.; Kim, Y.*; Lee, W. B.* († contributed equally)
Phys. Rev. E 2019, 100, 052502. DOI: 10.1103/PhysRevE.100.052502
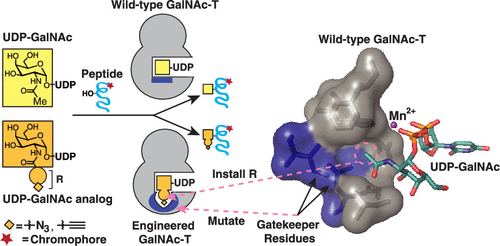
Engineering Orthogonal Polypeptide GalNAc-Transferase and UDP-Sugar Pairs.
Choi, J.†; Wagner, L. J. S.†; Timmermans, S. B. P. E.; Malaker, S. A.; Schumann, B.; Gray, M. A.; Debets, M. F.; Takashima, M.;Gehring, J.; Bertozzi, C. R.* († contributed equally)
J. Am. Chem. Soc. 2019, 141, 13442−13453. DOI: 10.1021/jacs.9b04695
Theoretical Study on the Toxicity of ‘Novichok’ Agent Candidates.
Jeong, K.*; Choi, J.
R. Soc. Open Sci. 2019, 6, 190414. DOI: 10.1098/rsos.190414
Transition Metal-Catalyzed Alkyl–Alkyl Bond Formation: Another Dimension in Cross-Coupling Chemistry.
Choi, J.; Fu, G. C.*
Science 2017, 356, eaaf7230. DOI: 10.1126/science.aaf7230
A General, Modular Method for the Catalytic Asymmetric Synthesis of Alkylboronate Esters.
Schmidt, J.; Choi, J.; Liu, A. T.; Slusarczyk, M.; Fu, G. C.*
Science 2016, 354, 1265−1269. DOI: 10.1126/science.aai8611
Enantioselective Decarboxylative Arylation of α-Amino Acids via the Merger of Photoredox and Nickel Catalysis.
Zuo, Z.†; Cong, H.†; Li, W; Choi, J.; Fu, G. C.*; MacMillan, D. W. C.* († contributed equally)
J. Am. Chem. Soc. 2016, 138, 1832−1835. DOI: 10.1021/jacs.5b13211
Stereoconvergent Arylations and Alkenylations of Unactivated Alkyl Electrophiles: The Catalytic
Enantioselective Synthesis of Secondary Sulfonamides and Sulfones.
Choi, J.; Martín-Gago, P.; Fu, G. C.*
J. Am. Chem. Soc. 2014, 136, 12161−12165 (spotlighted in JACS). DOI: 10.1021/ja506885s
A Versatile Approach to Ullmann C−N Couplings at RoomTemperature: New Families of Nucleophiles and
Electrophiles for Photoinduced, Copper-Catalyzed Processes.
Ziegler, D. T.; Choi, J.; Muñoz-Molina, J. M.; Bissember, A. C.; Peters, J. C.*; Fu, G. C.*
J. Am. Chem. Soc. 2013, 135, 13107−13112. DOI: 10.1021/ja4060806
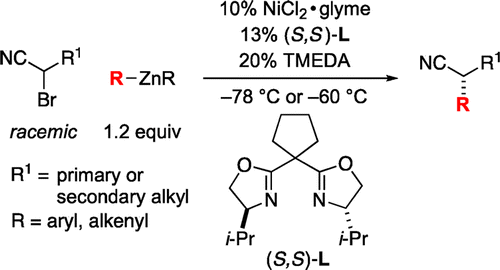
Catalytic Asymmetric Synthesis of Secondary Nitriles via Stereoconvergent Negishi Arylations and
Alkenylations of Racemic α-Bromonitriles.
Choi, J.; Fu, G. C.*
J. Am. Chem. Soc. 2012, 134, 9102−9105. DOI: 10.1021/ja303442q